
January 23, 2023
Lessons from horticulture: Pioneering the first antifungal vaccine
Anivive's work is gaining attention as the threat of systemic fungal infection grows

Anivive's work is gaining attention as the threat of systemic fungal infection grows
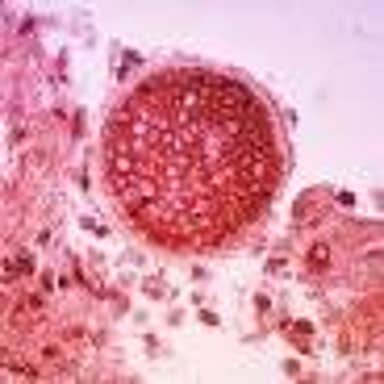
Anivive's work on an antifungal vaccine to prevent canine Valley Fever is referenced in this Wired article.

Anivive's work on an antifungal vaccine to prevent canine Valley Fever is referenced in this Freethink article.
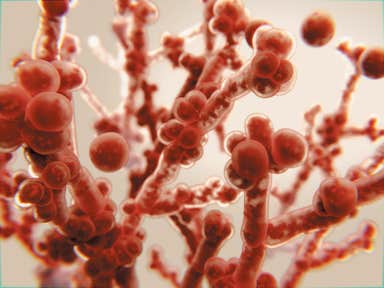
Anivive's work with Dr. John Galgiani and his research team at the Valley Fever Center for Excellence at the University of Arizona College of Medicine is gaining attention as the threat of systemic fungal diseases escalates globally.

Dr. David Bruyette, Anivive's CMO, talks about his company, a pharmaceutical startup looking for medical solutions for unmet needs in the veterinary space, that has created LAVERDIA™-CA1 (verdinexor), the first pill to treat cancer, in this case lymphoma in dogs, which may someday benefit humans, too.
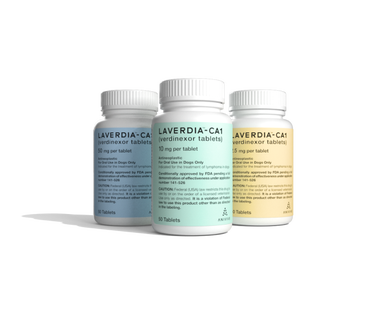
Anivive Lifesciences Inc. is taking its first pet-specific drug to market after 3.5 years and $5 million in research and development costs.

Medications from Pegasus Laboratories and Anivive Lifesciences earned conditional approvals.

Anivive Lifesciences, is working on a COVID-19 antiviral drug that’s inspired by cats, and it has new preclinical research findings to back up the project.

Anivive Lifesciences starts with unmet therapeutic needs and employs high-tech research methods to identify compounds that could meet those needs.