
Treatments that veterinarians
Specialty medicines
Shaping the future
of pet health
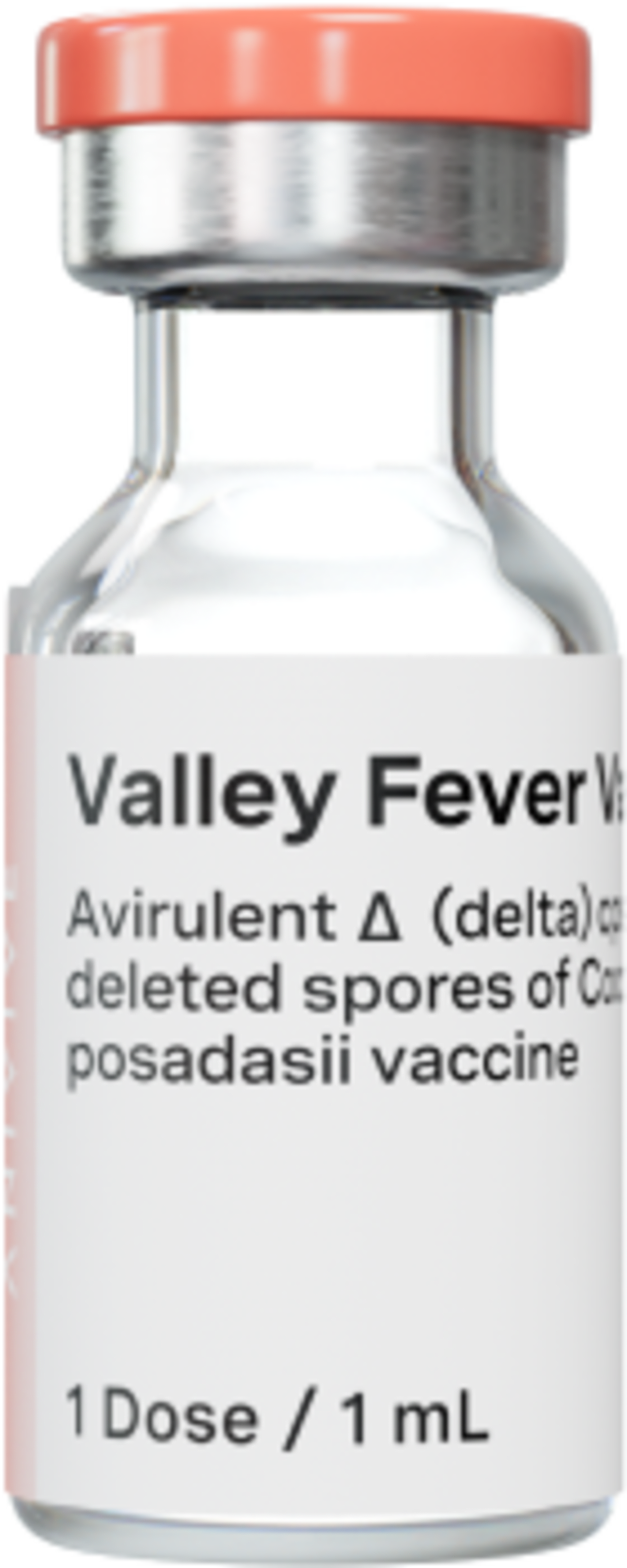
The first potential vaccine for a systemic fungal infection - in any species.
Investigational Coccidioidomycosis Vaccine
Canine Valley Fever
First-in-class Preventive
A potential first-ever vaccine for systemic fungal infections
Gene-deleted Strain
Deletion of disease-causing gene allows for use as a preventative vaccine
Standard Administration
Designed to be given as an injection—the same way dogs receive other vaccines
The first anti-viral with published efficacy for FIP in cats
Investigational Treatment (GC376)
Feline Coronavirus FIP
Targeted Antiviral
Designed to block coronavirus from replicating
Rapid Reversal of Symptoms
In feline clinical trials
1st Investigational Therapy
For FIP shown to be safe and effective in published studies
Investigational Treatment (eBAT)
Hemangiosarcoma
One of the more common cancers in dogs, accounting for approximately 20% of soft-tissue sarcomas and 5% of all non-skin tumors.
Investigational Treatment (eBAT)
Development Progress
Mid Stage
Our lead candidate is eBAT, a bispecific angiotoxin designed to specifically target hemangiosarcoma cells while reducing off target side effects.
Two studies with eBAT have demonstrated an increase in 6-month survival from <40% to approximately 70%. Further, 1 in 4 dogs were long term survivors, living more than 450 days after diagnosis.
Importantly, these studies also showed a single cycle of eBAT was as effective as repeated cycles with fewer adverse reactions.

Targeted Mechanism
Bispecific angiotoxin which binds to both EGFR and uPAR on tumor cells resulting in toxin release
Increase in Survival
Versus standard of care, as observed in clinical trials
Accelerated FDA Path
MUMS designation makes eBAT eligible for conditional approval
Development Milestones
FDA INAD File
Opened for eBAT
Complete
Safety and Dose Studies
Optimized dose
Complete
Pilot Field Efficacy Studies
Conducted Univ of Minnesota
Complete
Scaling Manufacturing
Capacity for CMC validation
Planning
Treatments. Vaccines.
Novel Applications.
Valley Fever
CPS1USDA
FIP
INAD I-013287
Blastomycosis
BAD1USDA
Hemangiosarcoma
INAD I-013225
Gingivitis
Lysine Decarboxylase
Osteosarcoma
INAD I-013511Products in development not approved by the FDA or USDA